HoPeNET
Establishing pathways for recruitment and retention of racial/ethnic groups underrepresented in clinical research (i.e., non-Hispanic Blacks in the Washington, DC metropolitan area).


Surveys
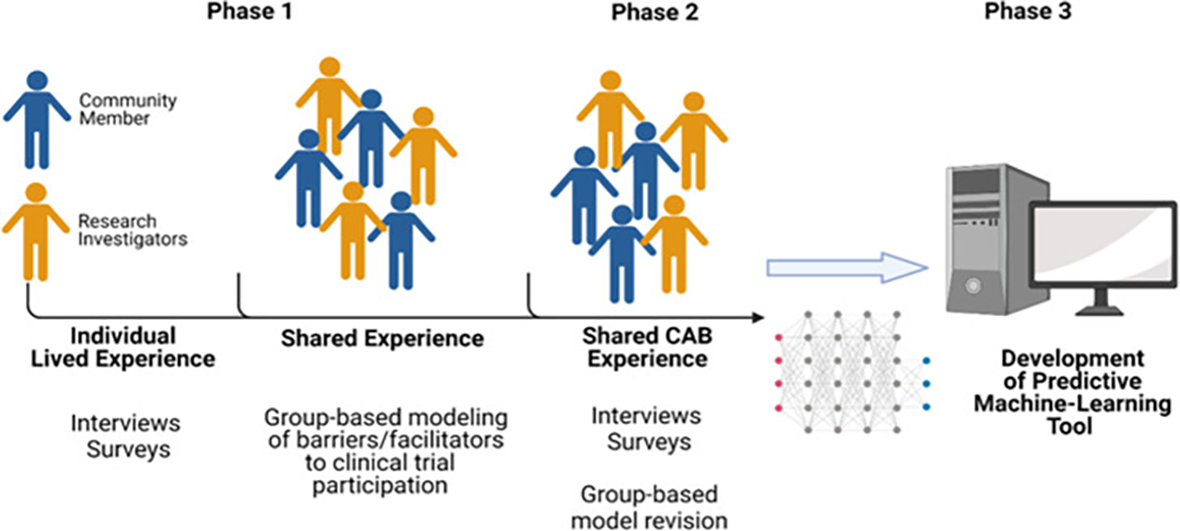
A preparticipation survey and one-on-one interviews will be used to capture perceptions and beliefs surrounding clinical trial participation.

Focus Groups
Three focus groups/workshops with (1) community members, (2) investigators and (3) both groups combined will be conducted.

Machine Learning
The implementation of data results for the development of the ML-based tool and outcome evaluation.
There are two main groups of potential participants for the HoPeNET CAB:
Graphical abstract of HoPeNET protocol
HoPeNET will aid in creating a predictive algorithmic tool to help increase African-American clinical trial participation.
Ethics and Dissemination
Participant confidentiality and privacy will be strictly maintained and held in trust by the participating investigators, and their staff. No information concerning the study, or the data will be released to any unauthorised third party without prior written approval of the Principal Investigator. The study data entry and study management systems used by research staff will be secured and password protected. At the end of the study, all records will continue to be kept in a secure location for as long a period as dictated by the reviewing institutional review board, institutional policies or sponsor requirements. We anticipate minimal risk for this study. However, we will ask participants to express their perceptions surrounding barriers and facilitators of clinical trial participation during the one-on-one interviews and focus group activities. We recognise that this activity may elicit emotional distress. Study participation will be voluntary and interviews can be stopped at any time. To be consistent with CBPR principles and to the stated programme evaluation, study findings will be disseminated to the HoPeNET CAB, presented at departmental and institutional levels at HU and the NIH IRP. We will also present our findings at national and international conferences, and peer-reviewed manuscripts from our project will also be submitted for publication.


